Business
Business model focused on both internal development and licensing
INTERNAL PRODUCT DEVELOPMENT
INTERNAL TECHNOLOGY DEVELOPMENT
EXTERNAL LICENSING AND CO-DEVELOPMENT
Oral
Parenteral
Drug Combinations
Multiple Industries
Clinical Trials and/or Asset Sales
Licensing, milestones, royalties
Multiple partners
Multiple solutions
Multiple products
Multiple industries
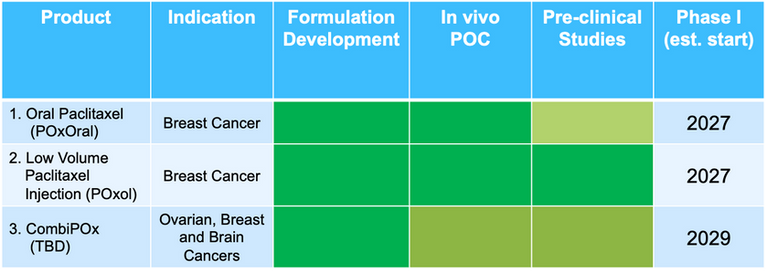
POx SOLUBILIZED PACLITAXEL IMPROVES UPON STANDARD OF CARE - ABRAXANE ®
-
POxOral: New IP filed 2024. Dramatic increase in oral bioavailability outperforming all other oral paclitaxel formulations. 505b2 regulatory approach possible to decrease development costs and time to approval.
-
POxol injection: Increases potency and convenience while decreasing cost over slow infusion required for Abraxane. Demonstrated to be 505b2 bioequivalent to Abraxane in primates.
-
CombiPOx: Simplifies development for Combination Therapies with multiple drugs to provide improved anti-tumor activity and greater convenience. Several indications and drug combinations in progress.